Summary
Inflammation reduces profitability, endangers health and product quality. Inflammation occurs frequently in intensive, modern animal production systems, particularly when routine antibiotics are being reduced. Controlling inflammation promises ROI in terms of feed conversion and health benefits to the livestock. This control is now the proposed mode of action (MOA) of commonly used antimicrobials in feed (AGPs). By managing triggers of inflammation and actively downregulating inflammation, excellent results can be achieved with alternatives to antibiotics in terms of productivity and even welfare. It is all about changing focus from anti-microbial effects to anti-inflammatory.
Where and when does inflammation occur?
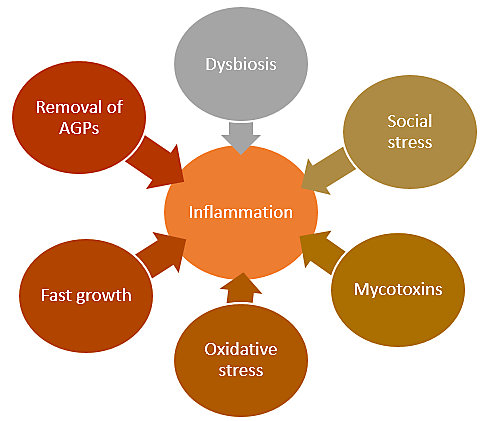
As livestock production has intensified across many countries, inflammation has become a common side effect. An increase has been observed in productions systems where routine antibiotic usage has been discontinued. It is a natural process and has been connected to desirable outcomes, such as controlled apoptosis, however, it can get out of hand. Inflammation becomes a challenge when the up- and down-regulations of the process are not well matched. There are different triggers that can start this process such as oxidative stress, certain mycotoxins, social stress, fast growth rates, and certainly dysbiosis of the gut microbiome. Several of these inflammation starting points also trigger each other (e.g. social stress leading to dysbiosis) which can lead to a vicious cycle of inflammation.
Once inflammation has gone beyond the normal level, it becomes very challenging for the animal to regulate it again back to normal levels. While inflammation has likely always posed a challenge in livestock production, its significance is certainly increasing in modern systems.